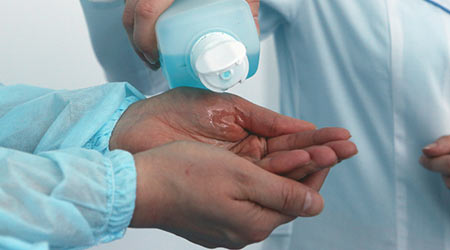
The Food and Drug Administration (FDA) recently announced it was removing 24 over-the-counter disinfectants or antimicrobial ingredients, including triclosan, that are being used in the medical setting, according to an article on the Beyond Pesticides website. The agency took this action because the chemical industry did not respond to a 2015 request for data to support a finding of “generally recognized as safe and effective (GRASE).”
The development follows a 2016 FDA decision to remove consumer soap products with triclosan for the same reason.
The most recent decision does not apply to six antiseptic compounds, following industry requests for more time to develop safety and efficacy data. The agency is leaving these most widely used compounds in these products, under chemical industry pressure, the article said.
“In response to requests from industry, the FDA has deferred final rulemaking for one year, subject to renewal, on six specific active ingredients that are the most commonly used in currently marketed OTC health care antiseptic products ‒ alcohol (ethanol), isopropyl alcohol, povidone-iodine, benzalkonium chloride, benzethonium chloride, and chloroxylenol (PCMX) – to provide manufacturers with more time to complete the scientific studies necessary to fill the data gaps identified so that the agency can make a safety and efficacy determination about these ingredients,” according to an FDA statement.
When the ruling was announced, Beyond Pesticides executive director Jay Feldman noted, “FDA’s decision to remove the antibacterial triclosan, found in liquid soaps, is a long time coming. The agency’s failure to regulate triclosan for nearly two decades ... put millions of people and the environment at unnecessary risk [of] toxic effects and elevated risk [of] other bacterial diseases."
Click here to read the full article.

 Celebrating BSCAI's 60th Anniversary eBook
Celebrating BSCAI's 60th Anniversary eBook The Down and Dirty on Cleaning in Virus Season
The Down and Dirty on Cleaning in Virus Season How Surfactant Use is Expanding in Commercial Cleaning
How Surfactant Use is Expanding in Commercial Cleaning